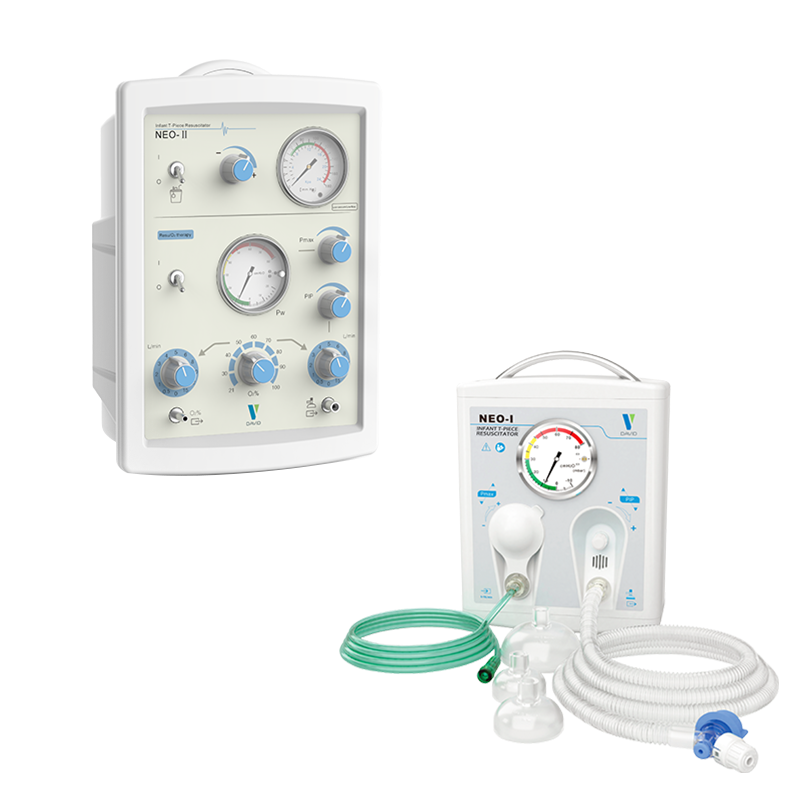
Description
- Applicable placesWidely used in delivery rooms, baby wards, transport processes, and neonatal intensive care units (NICUs)
- Gas driveGas driven control, no power supply required
- Easy to useReduce fatigue during resuscitation for healthcare professionals
- Design ScienceDesigned according to the latest clinical resuscitation guidelines of the American Academy of Pediatrics (APP, NRP) and the International Council on First Aid and Resuscitation (ILCOR).
- Recommended EquipmentThe latest clinical resuscitation guidelines recommend that medical institutions use this device, mainly because it can provide controlled and stable peak inspiratory pressure (PIP) and positive end-expiratory pressure (PEEP), ensure functional residual capacity (FRC), improve lung compliance, and provide safe and effective respiratory resuscitation and ventilation management for premature infants.






Reviews
There are no reviews yet.